January 29th, 2017
Welcome back to the Nerdy Teacher's Corner!
Mondays are always a really short class. This Monday was not much different. For today, I decided to teach students the easy way to do electron configuration. Now, of course they don't feel that this is always easy. However, it is much easier than trying to memorize the arrow diagram.
I'm going to be really honest here. When I showed it to them the first time I got a lot of confused looks. I had to reteach it because I felt that I didn't teach it properly the first time around. How it is explained in the rest of this blog is how I taught it the second time around.
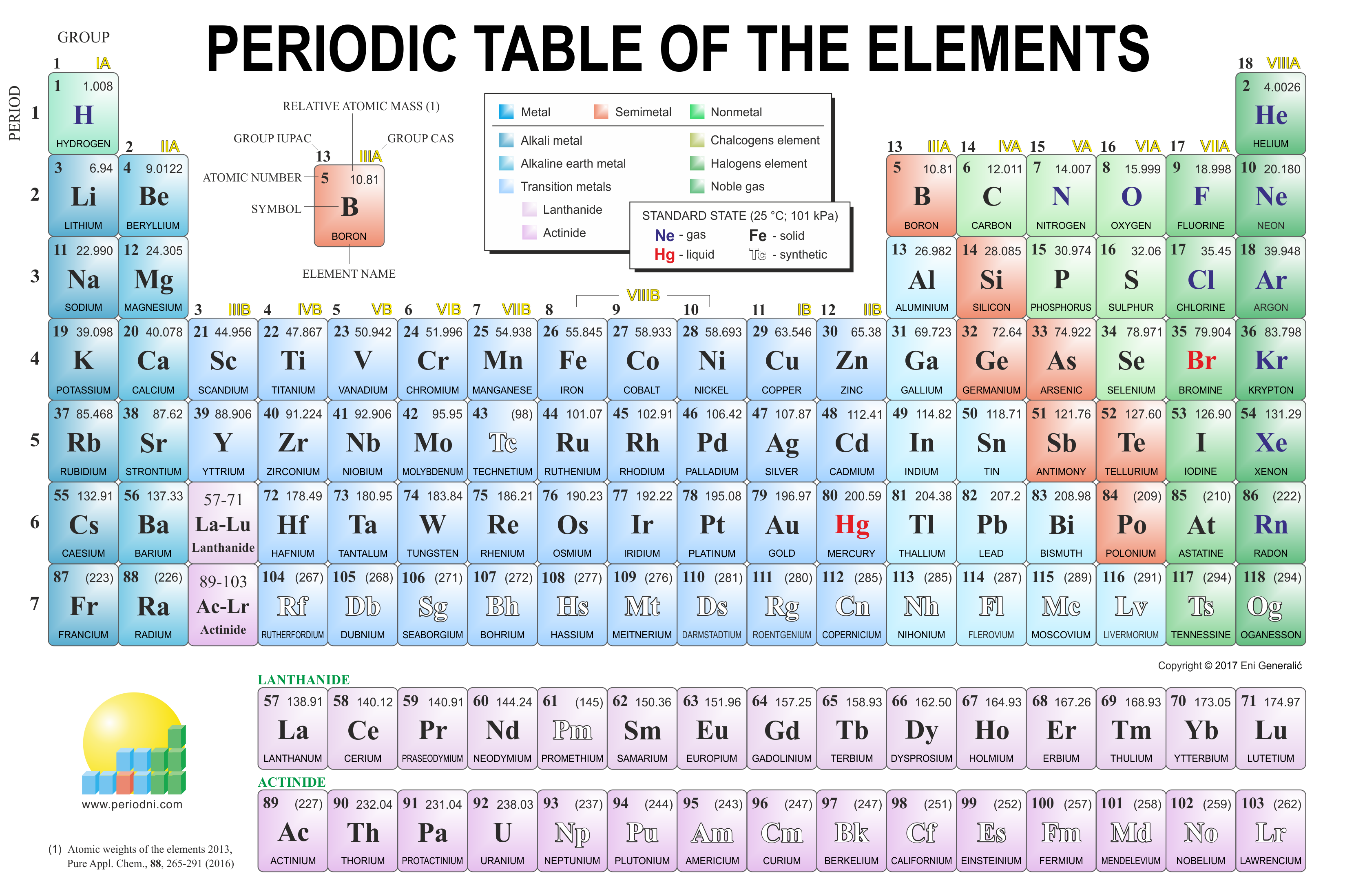
To start, we need to have our best friend, The Periodic Table of Elements. (Really, go get one right now!)
Okay, so first I had my students label each part of their periodic table. Group 1 and 2 both should have the s orbital on top. Group 13-18 (minus Helium) should be labeled with a p. Group 3-12 (Transition metals) should be labeled with d-1. And the Lanthanides and Actinides are labeled f-2. Like this:
Once the students have labeled their period table, we select an element. I like to pick one from the d orbitals simply because d is where students begin to get confused.
After picking the element, we circle it. Circling seems to show students exactly where they need to end up. Let's say in our case we select Iron. If you look at the Periodic Table, it should be element 26.
I told the kids, it's like going over to someone's house. You can't just cut across streets even though it's right behind your house, you have to just take the streets over to the house whether you're walking, driving, or biking. Let's start the beginning of the Periodic Table.
At this point, students have numbered each period on their Periodic Table. We can use this for energy level as well. Let's start. The first step where Hydrogen is at is our starting position. What energy level is that? 1. Now, what orbital is it in? s. Okay, we have 1s. How many electrons in an s orbital? Two. That's Hydrogen and Helium. We took 2 steps. We now have 1s to the 2nd power. 1s^2. Are we at Iron yet? No. We're only at Helium! Let's go down. Energy level? Orbital? How many steps? 2s^2. Beryllium. If we go across the periodic table we hit Boron. Energy level? Orbital? How many steps? 2p^6. So on and so forth. It's a little hard to explain over a blog. What I will be trying to do is uploading videos on how to do this. However, that's not something I have yet.
Once we completed this explanation, light bulbs seemed to go off everywhere! I took this opportunity to hand out the electron configuration practice worksheet that will be turned in and an additional worksheet that will be extra credit. You can find that on my Resources page
Again, because Mondays are really short, we don't get through a lot during the day. The rest of the time, I let my students work on the electron configuration while I walked around trying to help.
That's all I have for you today! Join me next time on the Nerdy Teacher's Corner!
That's all I have for you today! Join me next time on the Nerdy Teacher's Corner!
No comments:
Post a Comment